Glomb Lab
In vivo Dynamics of the Neuronal Cytoskeleton
Neurons contain a complex and highly specialized cytoskeleton to function throughout life. The cytoskeleton protects the neuron from extrinsic deformations caused by body movement, scaffolds membrane-, signaling proteins and ion channels as well as organelles and mediates their transport. Consequently, mutations in its core components result in neurodegenerative conditions and are affiliated with motorial and intellectual disabilities in human patients. Therefore, understanding how the neuronal cytoskeleton forms and how it is organized to support the neuron throughout lifetime is essential but remains poorly understood. We combine live cell imaging in living nematodes, spatiotemporally controlled labeling strategies, genetics and biochemistry to understand how the neuronal cytoskeleton forms with a focus on the membrane associated periodic skeleton.
Contact
Dr rer. nat. Oliver Glomb
Working Group Leader
Future directions of the Lab
How are cytoskeletal proteins delivered to the axon?
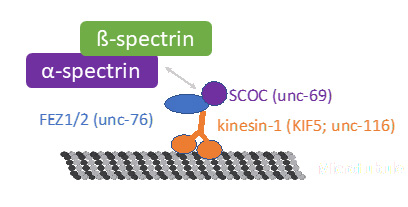
Studying the transport of cytoskeletal proteins is technical challenging, especially in vivo, given their high overall abundance throughout the entire neurite, so that the transport can not be studied by conventional fluorescent labeling approaches. We recently developed a toolbox to spatiotemporally control the labeling of cytoskeletal components and utilized it to describe the transport of spectrin, which forms the central building block of the membrane associated periodic skeleton. To better understand the transport mechanism that conveys cytoskeletal proteins, we want to further dissect the molecular composition of the spectrin transport complex and apply our labeling approach to other cytoskeletal components. See our recent paper: DOI: 10.1016/j.devcel.2023.08.031.
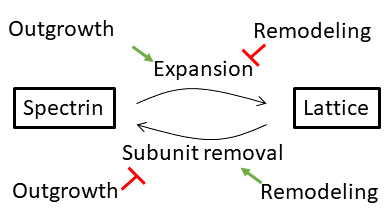
How does the cytoskeleton scale with axonal growth?
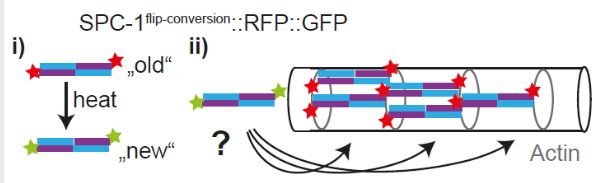
We recently identified assembly sites from which novel spectrin molecules extend the outgrowing cytoskeletal lattice. To understand how this expansion is scaled to the growth of the neurite and organism, we want to characterize the molecular composition of assembly sites. See our recent preprint: DOI: 10.1101/2022.05.09.491207.
How do cytoskeletal proteins turn over?
The proteolytic degradation of spectrin can lead to neurodegeneration and thus needs to be tightly controlled. We believe that understanding the mechanisms that regulate spectrin turn-over are of importance to complement our understanding of the progression of neuronal ageing. Our labeling strategy allows us to locate where and how the spectrin lattice turns over.