
Hirt Lab
Suitability of Aminolipin® as a fixative for histological and microscopic analysis
The fixation agent Aminolipin® was developed at the Institute of Clinical Anatomy and Cell Analysis as a substitute for the carcinogenic formaldehyde and is currently evaluated extensively for use in anatomical macroscopy. Proof of principle has also been provided for use in microscopy/advanced microscopy. Works on efficacy, functionality and hazard potential for the user were funded by two BMBF programmes and by the German Social Accident Insurance (Deutsche Gesetzliche Unfallversicherung/DGUV). Extensive biochemical, molecular biological and anatomical studies demonstrated the excellent fixation and preservation effectiveness of Aminolipin® for human whole-body preparations. Certified tests on the hazard potential prove, among other things, that the new active ingredient is non-volatile, non-mutagenic and non-genotoxic, unlike formaldehyde. Thanks to the newly discovered efficient inhibition of proteases relevant for autolysis and its comprehensive antimicrobial spectrum of activity, Aminolipin® has the potential to replace formaldehyde for the fixation and preservation of biological materials. Based on initial promising histological studies, a further research focus will be to extensively test Aminolipin-containing fixation solutions for their suitability in the microscopic analysis of cells and tissues.
Contact
Prof. Dr Bernhard Hirt, MD
Director
Key Methods of the Lab
Molecular and cell-biological analysis, fixative chemicals and solutions, development of histological methods, Advanced Microscopy
The Aminolipin® Principle
Aminolipin® is a propylene diamine derivative containing a pyrrolidone ring and a carbon tail.
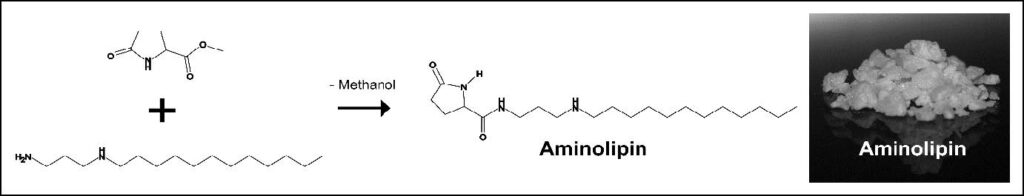
Aminolipin® is a fundamentally novel biocidal agent that is highly effective in inhibiting functional proteins. It is non-genotoxic, non-mutagenic and non-volatile, resulting in an improved safety profile. Aminolipin® is classified as readily and rapidly biodegradable and has a favourable ecotoxicological profile. It is currently the only biocide awaiting authorisation under the European Biocidal Products Regulation (BPR) (EU) No. 528/2012.
